 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 1 doi: 10.5376/ijmms.2024.14.0004
Received: 01 Mar., 2024 Accepted: 10 Mar., 2024 Published: 12 Mar., 2024
Yan S.D., 2024, Glucocorticoid receptor signaling: intricacies and therapeutic opportunities, International Journal of Molecular Medical Science, 14(1): 24-28 (doi: 10.5376/ijmms.2024.14.0004)
The paper "Glucocorticoid receiver signing: intricacies and thermal opportunities" published in the journal Trends in Biochemical Sciences on February 29, 2024, authored by Dorien Clarisse, Laura Van Moortel, Chlo é Van Leene, Kris Gevaert, Karolin De Bosscher, and others from the University of Bridget et al. This study delves into the signaling mechanism of glucocorticoid receptors (GR) and their potential therapeutic applications. GR, as a nuclear receptor (NR), plays a crucial role in regulating the treatment of inflammatory diseases and certain cancers. By reviewing the basic biological characteristics of GR, including its activation and nuclear translocation mechanisms in cells, this study discusses in detail the interaction between GR and chromatin, revealing how it regulates downstream gene expression by directly binding to DNA responsive elements or interacting with other transcription factors. In addition, new concepts in GR signaling were explored, such as the recruitment of co regulatory factors, the role of aggregate formation, and strategies to enhance therapeutic efficacy through interaction with other nuclear receptors. By integrating multiple technologies to study the conformation and modification of chromatin, we can gain a deeper understanding of the cell-specific effects of glucocorticoids (GCs), providing a new perspective for developing GR ligands and therapeutic strategies with fewer side effects. This study not only provides valuable insights into the complex role of GR in cells, but also provides a theoretical basis for the development of new therapeutic methods in the future, especially in research aimed at reducing treatment-related side effects while improving efficacy.
1 Experimental Data Analysis
This study provides a detailed description of the structure of GR, including its inter domain communication when binding to different ligands, and how it affects the interaction between GR and chromatin. By integrating technology to study chromosome conformation, accessibility, modification, and recruitment of cofactors, we can gain a deeper understanding of the cell-specific effects of glucocorticoids (GCs). Research has found that dimerization and allosteric communication in the GR field are crucial for the efficacy of glucocorticoids, and chromosome remodeling complexes are crucial for fine-tuning GR activity.
Figure 1 shows the diverse mechanisms of action of glucocorticoid receptors (GR). GR is mainly located in the cytoplasm when ligands are not bound, and exists in a partially unfolded state, preventing ligand binding through the Hsp70-Hsp40 chaperone complex. Subsequently, GR will undergo a series of complex transformations, ultimately forming an Hsp90-GR-FKBP52 nuclear transfer complex that can transfer to the nucleus. As a transcription factor, GR can mediate gene activation or inhibition. The inhibitory effect is usually achieved through interaction with other transcription factors, such as NF-κB The interaction between B and AP-1 is achieved, thereby inhibiting their activity. The mechanisms by which GR activates and inhibits gene expression are diverse, including direct DNA binding and indirect mediation or isolation of transcription factors through other transcription factors.
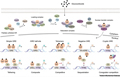 Figure 1 Diverse action mechanisms of the glucocorticoid receptor (GR) |
Figure 2 shows the isomeric communication within the glucocorticoid receptor (GR) structure and its new dimeric interface. GR consists of four structural domains: N-terminal domain (NTD), DNA binding domain (DBD), hinge region (HR), and ligand binding domain (LBD). DNA is considered an isomeric regulator of DBD conformation. Similarly, the binding of different ligands and co modulators also alters the conformation of LBD in different ways. In LBD, there are ligand and co regulatory binding pockets, and there is bidirectional communication between them. In addition, after ligand binding, domain to domain communication also occurs from LBD to DBD. The repositioning of the R611 site has expanded the pocket size, allowing for the binding of larger molecules. The A458 (DBD) and I628 (LBD) residues are crucial at the dimer interface, and dual mutations can lead to complete loss of function. The newly proposed LBD dimer exhibits a head to tail structure. There are still doubts about higher-level oligomeric states of GR (such as tetramers or higher), but a possible explanation is that they aggregate distant glucocorticoid binding sequences (GBSs) together.
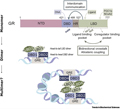 Figure 2 Allosteric communication within the GR structure and its new dimer interface |
Figure 3 illustrates the complex process of glucocorticoids (GCs) regulating gene expression through their receptors (GR). Under normal circumstances, GR activation can bind to glucocorticoid responsive elements (GREs) on genes, recruit co activators NCOAs and histone acetyltransferases (such as CBP and p300), as well as SWI/SNF complexes, leading to chromatin remodeling. This process promotes the recruitment of BRD4, mediators, and RNA polymerase II pre initiation complexes (PICs), promoting the transcription initiation and extension of downstream target genes. Under inflammatory conditions, NF-κB The p65 subunit of B will bind to its reactive element(κBREs, downstream gene transcription is activated. After GCs treatment, GR (monomers or dimers) recruit NCOR and HDAC proteins by connecting to p65, thereby inhibiting the binding of p-TEFb and recruitment of NELF, leading to transcriptional elongation arrest (B), or chromatin deacetylation by interacting with HDAC proteins, thereby inhibiting BRD4 recruitment, PIC assembly, and transcriptional initiation (C).
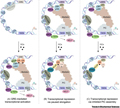 Figure 3 GR complex assembly |
Figure 4 explores the therapeutic opportunities brought about by the interaction (crosstalk) between glucocorticoid receptors (GR) and other nuclear receptors (NR). In estrogen receptor (ER) positive breast cancer, GR agonist combined with selective ER modulator may improve the treatment response. For triple negative breast cancer (TNBC), GR antagonists may be required, because GR may have a proliferative effect in the absence of ER. In prostate cancer sensitive to the androgen receptor (AR) antagonist enzalutamide (Enz), GR antagonists may prevent GR from replacing AR to regulate gene expression. In the presence of enzalutamide resistance, inhibiting specific co regulatory factors may sensitize prostate cancer cells to enzalutamide. In multiple myeloma (MM), the communication between GR and mineralocorticoid receptor (MR) can be treated by binding GR agonists and MR antagonists. In acute lymphocytic leukemia (ALL), ERRβ Agonists or LRH-1 antagonists may sensitize ALL cells to GR agonist induced cell killing. Finally, the anti-inflammatory activity of GR agonists may be related to PPARα Or PPARγ Enhanced when used in combination with agonists. These therapeutic opportunities reveal that fine-tuning GR and its related nuclear receptor communication can provide new therapeutic strategies.
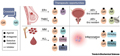 Figure 4 Therapeutic opportunities arising from GR crosstalk |
2 Analysis of Research Findings
This study explores the structural dynamics of glucocorticoid receptors (GR) and the allosteric effects after ligand binding, revealing the complexity of GR signaling and its regulatory mechanisms on specific gene expression. Specifically, the dynamic conformational changes caused by the interaction between GR and different ligands and co regulatory factors not only demonstrate the diversity of GR activity, but also provide a new perspective for understanding its role in cell specific gene regulation. In addition, the atypical dimerization pattern of GR ligand binding domain (LBD) and its interactions with DNA and other proteins further demonstrate the complexity of GR mediated transcriptional regulation. These findings are of great significance for a deeper understanding of the role of GR in physiological and pathological processes, as well as for the development of novel therapies for GR.
3 Evaluation of the Research
This study provides a profound insight into the signaling mechanism of glucocorticoid receptor (GR) by combining high-resolution structural analysis and biochemical techniques, particularly in its interaction with chromatin and ligand binding induced allosteric effects. By revealing the dynamic allosteric communication between GR ligand binding domain (LBD) and DNA binding domain (DBD), and how it affects the regulatory role of GR on target genes, this work provides an important molecular basis for understanding the disease mechanisms mediated by GR and developing new GR targeted drugs. However, although these findings deepen our understanding of GR activity regulation, further research and validation are needed to fully utilize this knowledge to develop GR agonists and antagonists with selectivity and fewer side effects.
4 Conclusions
This study delves into the complex mechanisms of glucocorticoid receptor (GR) signaling and its potential applications in the treatment of inflammation and certain cancers. Special attention was paid to the interaction between GR and chromatin, conformational communication in GR structure, and bidirectional communication between ligands and co regulatory factors. Through structural and molecular biology methods, novel insights into the atypical dimerization patterns of GR ligand binding domains (LBD) and their impact on GR function have been revealed, providing possibilities for the development of novel GR ligands with specific therapeutic effects and reduced side effects. These findings are of great significance for understanding the mechanism of action of GR in cells and developing new therapeutic strategies, especially in the pursuit of more precise and safe drug therapies.
5 Access the Full Text
Clarisse D, Van Moortel L, Van Leene C, et al. Glucocorticoid receptor signaling: intricacies and therapeutic opportunities[J]. Trends in Biochemical Sciences, 2024. https://doi.org/10.1016/j.tibs.2024.01.012.
Acknowledgments
I am particularly grateful to Trends in Biological Sciences for implementing an open access strategy, which allows researchers, students, and other readership groups to download, research, and share cutting-edge scientific papers for free. This strategy has greatly promoted the widespread dissemination of scientific knowledge and provided extremely rich resources for science enthusiasts worldwide. The open access model of Trends in Biological Sciences undoubtedly accelerates scientific progress and enhances public understanding and interest in scientific research. I am sincerely grateful for the tremendous contribution this magazine has made to the scientific community and society, and I look forward to further exploring the rich knowledge of science through this platform in the future.
. PDF(551KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Shudan Yan
Related articles
. Intricacies
. Therapeutic opportunities
Tools
. Email to a friend
. Post a comment